Emissions Control
Task 1 Team
-

Prof. Peter Styring
University of Sheffield
-

Prof. Enrico Andreoli
Swansea University
-

Dr George Dowson
University of Sheffield
-

Dr Craig Armstrong
Swansea University
Introduction
Steelmaking process and emission gases are a source of carbon dioxide from which to manufacture chemicals. The future availability of fossil-free chemicals is linked to hard-to-decarbonise processes including those of the foundation industries. This is particularly true for carbon-intensive BF-BOF steelmaking emitting ca. 1.9 tCO2/t crude steel, but also for lower carbon emitting EAF still releasing ca. 0.1 tCO2/t steel (0.3 tCO2/t steel when adding electricity CO2 intensity). The opportunity is to integrate CO2 capture and utilisation technologies in steel manufacturing processes to deliver added value in collaboration with the chemical industry.
The overall aim of Task 1 was to reduce steelmaking carbon emissions by capturing and converting CO2 into commercially valuable products. Valuable products made from CO2 lower the overall cost of carbon capture and sequestration, creating virtuous integration between steel and chemical manufacturing. CO2-derived chemicals and fuels are interesting for their added value and in some cases, large market scale, allowing a future circular economy based on the recycling of carbon dioxide. This approach is essential since steelmaking processes will continue generating CO2 while transitioning and deploying technologies with a lower carbon footprint.
Outcomes
One of the original aims of the project was to tackle BF-BOF process gases and heat/power generation (combustion of natural gas) carbon emissions. Whilst the idea of obtaining higher calorific value CO/H2 enriched gas streams for use in the integrated steelworks remains a valid transitional approach, there has been a shift towards EAF emissions with substantially different gas compositions. The direct CO2 emissions of a typical EAF are dependent on how much fossil fuel (natural gas, or even coal) is used as an energy source, accounting for 40%-70% of overall emissions. Substituting hydrogen for fossil fuel the process generates CO2 from graphite electrode consumption and from the thermal decomposition of additives such as CaCO3. As a result, CO, CO2, SOx and NOx are present in the emission of EAF steelmaking. The impact of contaminants such as sulphur and nitrogen oxide must be considered in the context of carbon capture and utilisation.
Since the inception of Task 1, we made significant progress in developing and scaling both carbon capture and utilisation technologies.
The FluRefin carbon dioxide capture and refining system has been developed from being a small laboratory scale unit to a ca. 0.1 t/d mobile capture unit, with considerable in-kind financial and engineering support from AESSEAL in Rotherham. This has put the technology approximately 5 years ahead of schedule. Consequently, we have filed a GB Patent Application in 2022 and now a PCT Application in January 2023. Sheffield has now issued an exclusive license to CCU International Limited in Scotland to begin the commercialisation of FluRefin. Field trials are being prepared for the deployment of the system at steelmaking sites and the refined gases will be converted to a number of value-added products including ethanol, methanol and DME for use as synthetic transport fuels. The utilisation reactors are currently being constructed and recommissioned.
The FluRefin technology was a short-listed Finalist at The Engineer Awards: Collaborate to Innovate. We were awarded the only Highly Commended Award at the ceremony in London on 23 February 2023. The technology was also announced as the winner in the Engineering Impact category at the Engineering & Manufacturing Awards in September 2023.
Figure 1. Field trials of FluRefin
Figure 2. Peter Styring & George Dowson at the Flurefin field trial with Ed Heath-Whyte at Liberty Steel
In parallel to SUSTAIN, we have deployed a state-of-the-art Pressure Swing Adsorption (PSA) unit at Rockwool Ltd, in their Bridgend manufacturing site, as a part of the RICE project. Since the inception of SUSTAIN Task 1, the alignment of research and innovation efforts of RICE has translated into the prospect of operating the PSA unit in the manufacturing sites of our steelmaking partners. Now that the PSA unit has been deployed, there is the opportunity to relocate and install the unit when available where most appropriate for our SUSTAIN industrial partners.
Our CO2 utilisation technologies have matured during the last year. We are now implementing in the laboratory, large-scale electrochemical conversion solutions to bench-scale carbon dioxide electrolysers to generate chemical products like ethylene. Ethylene is of great interest given the global production scale (150Mt C2H4/year) and the potential to replace the use of fossil carbon whilst avoiding vast amounts of carbon emissions (250Mt CO2/ year). We developed new CO2 electrolysers integrating technical solutions from the largest electrolysis process in the world, the chloralkali process. The purpose of this novel approach is to deliver stable CO2 electrolysis, as robust as the chloralkali process achieving the thousands of operational hours necessary for commercial implementation. Currently, we have designed and built a 30 cm³ CO2 electrolyser (10+ times scale-up) to include the falling film technology responsible for the long-term stable operation of the chloralkali process. In parallel to that, we have been making in the laboratory CO2 reduction gas diffusion electrodes following industrial manufacturing procedures in view of achieving robust and scalable ethylene production performance. We linked up with Industrie De Nora, a global leader in chloralkali electrode technology, and maintain a common interest in discussing collaborative opportunities once our falling film electrolysers become operational.
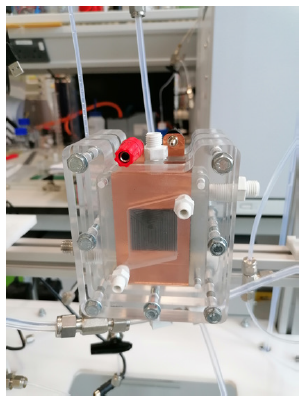
Figure 3. Enrico Andreoli and the vertical CO2 electrolysis rig
Figure 4. Laboratory scale CO2 reduction gas diffusion electrodes
Impact
The FluRefin system developed at Sheffield has now been licensed for commercial development and has had follow-on funding from Innovate UK Transforming Foundation Industries Challenge fund to develop a larger 1 t/d system, housed in a 20’ ISO shipping container. The Flue2Chem project also includes current partners Tata as well as 12 other major global companies including Unilever, P&G, BASF, JM and others.
The integration of carbon utilisation technologies requires close collaboration between steelmakers and chemicals manufacturers with a shared vision of a transformed and merged industry where CO2 from steel production is in the carbon feedstock for chemical production. The industrial impact of this approach is transformative in the way that a sustainable circular economy is achieved whilst decarbonising steelmaking and defossilising chemicals manufacturing. A key barrier to implementation is establishing executive communication between steel and chemical industries to create and support a bold and forward-looking vision to grant investments in new CO2 utilisation technologies. In the case of Task 1 CO2 electrolysis, we need to address practical issues in the laboratory with scalability in mind. For example, we are currently testing our new CO2 falling film electrolyser fully aware that the resulting technology will have to be transferred to the pilot scale (10-100 times larger electrodes). Currently, we work in the laboratory to de-risk the falling film technology to then embark on scale-up activities with our industry partners.
Next Steps
Innovate UK funded Flue2Chem will allow us to build a 1 t/d system and then we will work with commercial partners to move to a standard 10 t/d modular capture facility. Within Task 1 of SUSTAIN we need to develop the connectivity between the capture and utilisation units and to develop new catalysts and processes. A big challenge will be scale matching between the volume of captured CO2 and the ability to convert it all without re-emitting any excess to the atmosphere. Therefore, we are adding a downstream liquefaction facility to allow us to store captured CO2 for subsequent use and also for public engagement activities, allowing people to see pure liquid CO2 being formed.
The next stage of development of CO2 electrolysis is to demonstrate long-term operation with sustained production of ethylene also in the presence of gas feed impurities. We need to understand and minimise the impact of gas contaminants (SOx, NOx), optimise the process for high energy and product formation efficiency, and establish a channel of communication between steelmaking partners and the chemicals industry to participate and invest in de-risking our CO2 falling film technology.